Nitrogen didn’t start out naughty. It comes from a good home: a blue planet that I hear is very nice. It constitutes 78 percent of our planet’s atmosphere and enjoys cosmic notoriety as the sixth most abundant element in the universe. Way to go number 7 (check your periodic table). Life wouldn’t be much good without it, and it finds its way into many products: food, fertilizer, explosives, refrigerants, metals, and jet and rocket propellants. If you’ve had the good fortune to witness the aurora borealis, you have Nitrogen to thank, at least in part, for the experience. Oh. . .but Nitrogen has a naughty side.
Nitrogen derives its name from a combination of the Greek nitron (sodium carbonate or soda) plus the French gene (producing or giving birth to). In the 1770s it was also called mephitic air, meaning air devoid of oxygen (Blaszczak-Boxe, 2017; Sanderson, 2019).
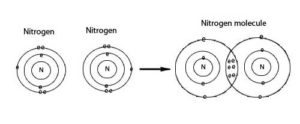
Nitrogen in the atmosphere is N2: two nitrogen atoms held together by the notorious “triple bond.” This is one mighty bond – the chemical equivalent of the sword in the stone. Due to this bond, N2 is basically non-reactive. It hangs out in the atmosphere until one of two natural forces can extract the sword from the stone (i.e., convert N2 to more reactive forms): (1) lightening, and (2) fixation by legumes and bacteria that converts N2 from the atmosphere into more biologically-available forms. The resulting reactive forms of N include ammonia, nitrogen oxides, urea, proteins, and nucleic acids (Galloway et al., 2003).
Graphic: The amazing triple bond of two N atoms to form N2. Source: Washington State University, Center for Sustaining Agriculture and Natural Resources: http://csanr.wsu.edu/the-reactive-nitrogen-wicked-problem/
Not surprisingly, humans have affected the creation of reactive N through the chemical processes that produce fertilizers and other products. The most widespread of these is known as the Habor-Bosch process: an artificial N fixation process that combines N2 and hydrogen at high pressures to produce ammonia. The process allowed ammonia to be produced economically for agricultural and industrial applications. Both of its namesakes – Fritz Haber and Carl Bosch – received Nobel Prizes in 1918 and 1931, respectively (Encyclopedia Britannica, 2018). One can imagine the contribution this innovation bestowed on a growing and hungry world.
In addition to Habor-Bosch, combustion of fossil fuels contributes to the reactive N issue, as it converts N2 primarily to reactive nitrates.
Now, here is the naughty part. Prior to its fabrication by humans, the production of reactive N was largely in balance with the consumption of N by microorganisms and vegetation, meaning that reactive N did not accumulate in our ecosystems. However, since the turn of the 20th century, production of reactive N has increased 17-fold in the U.S. alone (Frear, 2014), with agricultural applications accounting for the majority of this increase.
A study conducted by the University of Alabama of nutrients sequestered in Chesapeake Bay oyster shells showed a dramatic increase in nitrogen beginning in the early 1800s. Oysters have provided a handy and stationary gauge for nutrient levels in the water through time; as filter feeders, the oysters transfer nutrients from the filtered water into their shells. The time period of dramatic increase coincided with industrialization, population growth, clearing of forests, and plowing of fields. This increase in oyster shell N has continued its exponential growth through present times (Smedinghoff, 2017).
I believe readers of this post are familiar with the effects of N in our aquatic and coastal systems, such as the Chesapeake Bay and Gulf of Mexico: eutrophication, acidification, algal blooms, and low oxygen levels causing dead zones. That, as it turns out, is only part of the story of naughty N.
University of Virginia professor James N. Galloway and his colleagues have developed the “Nitrogen Cascade” theory (Galloway et al., 2003). The theory describes a process by which excess N is deposited in, stored, and transferred between the atmosphere and terrestrial and aquatic systems, as it “cascades” from one environmental sector to the next.
As an example of the cascade affect, a single atom of reactive N created through fossil fuel combustion can, in sequence:
- Increase atmospheric ozone and smog
- Decrease atmospheric visibility
- Increase the acidity of precipitation and, as a result, soils and aquatic systems
- Decrease biodiversity
- Decrease productivity of forests and croplands
- Promote eutrophication in water bodies, especially in coastal areas
- Increase N in groundwater
- Be converted to nitrous oxide and emitted back into the atmosphere
- Contribute to greenhouse warming
- Impact human health
(Galloway et al., 2003; Frear, 2014)
That’s one naughty atom!
The cascade is not exactly a linear process, because each stop-over along the cascade (e.g., atmosphere, forests, grasslands wetlands, marine ecosystems) has variable abilities to store N temporarily, transfer it to other ecosystems, and/or convert it back to N2 through denitrification. For instance, N can be stored in forests for years or even centuries, but has an impact while residing there.
The take-home, however, is that the production of reactive N is far out of equilibrium with the capacity of the earth’s systems to process it. This essential point has led Nobel Peace Prize recipient Dr. Otto Doering to declare reactive N as one of the world’s Wicked Problems, on par with affordable health care and climate change (Frear, 2014). This Wicked Problem just happens to be hiding in plain sight as N shape-shifts between its various chemical forms within earth’s ecosystems.
Several solutions have been proffered. The most obvious of these are to use N more efficiently and to restore and expand ecosystems that can convert reactive N back to atmospheric N2. The Chesapeake Bay efforts to restore aquatic grasses and coastal wetlands is an example (EcoCheck et al., 2010).
Some researchers have reached for more exotic solutions, such as promoting super microbes that specialize in oxidizing ammonia (Blaszczak-Boxe, 2017), which, to me, sounds like one of those scary microbes-out-of-control movies.
We certainly can’t live without Nitrogen. Our issue is living with too much of it. We have contributed to the delinquency of N; now it is time to apply some disciplinary actions.
Note: Dr. Galloway has continued to publish numerous articles about N since the Nitrogen Cascade paper cited here. See his page for more information: https://www.evsc.virginia.edu/galloway-james-n/
Let me know your personal experiences with Nitrogen (or any other element): [email protected]
References
Blaszczak-Boxe, A. Facts About Nitrogen. Live Science, September 28, 2017. https://www.livescience.com/28726-nitrogen.html
Chesapeake EcoCheck (NOAA-EMCES), Maryland Department of Natural Resources, University of Maryland Center for Environmental Science, USGS, Old Dominion University, Chesapeake Bay Program. Nitrogen in the Chesapeake Bay: A Retrospective. August 2010. https://ian.umces.edu/pdfs/ian_newsletter_275.pdf
Encyclopedia Britannica. Haber-Bosch process. October 28, 2018. https://www.britannica.com/technology/Haber-Bosch-process
Frear, C. The Reactive Nitrogen “Wicked Problem” – critical nutrient, disastrous pollutant. Washington State University, Center for Sustaining Agriculture and Natural Resources, August 11, 2014. http://csanr.wsu.edu/the-reactive-nitrogen-wicked-problem/
Galloway, J.N., Aber, J.D., Erisman, J.W., Seitzinger, S.P., Howarth, R.W., Cowling, E.B., and Cosby, B.J. 2003. The Nitrogen Cascade. BioScience, April 2003, Vol. 53 No. 4, pgs. 341-356.
Sanderson, R.T., Nitrogen: Chemical Element. Encyclopedia Britannica. Last updated February 1, 2019. https://www.britannica.com/science/nitrogen
Smedinghoff, J. 2017. Oyster shells show evidence of early human pollution of Chesapeake Bay. Chesapeake Bay Program, April 24, 2017. https://www.chesapeakebay.net/news/blog/oyster_shells_show_evidence_of_early_human_pollution_of_chesapeake_bay